Quality, the cornerstone of your industrial projects
In the healthcare industry, every facility, every process and every piece of equipment must meet the highest standards of quality and compliance. That is why, at Projipharm Ingénierie, we incorporate regulatory requirements from the earliest stages of design.
EXPERTISE ROOTED IN CONSULTING AND REGULATIONS
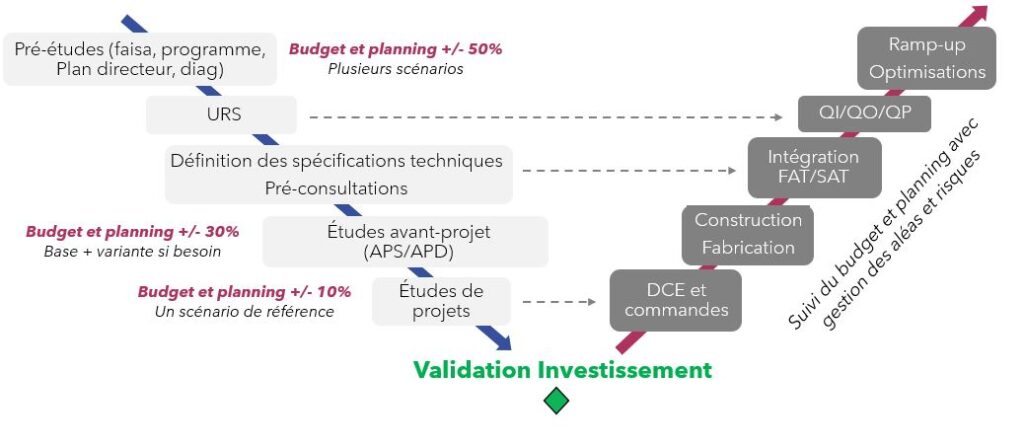
With a background in consulting, we have in-depth knowledge of the quality processes and regulatory requirements that govern the healthcare industry. Whether for a new building, laboratory or production line, we structure each project around the key stages of qualification and validation.
✔ Consideration of regulations and guidelines from the design stage onwards,
✔ Impact of URS on QI/QO/QP qualifications,
✔ Ensuring a smooth transition to commissioning,
✔ Documentation drafting and management: we adapt to your standards or provide our own document templates,
✔ Anticipation of FATs and SATs to ensure equipment compliance before on-site integration.
A DIRECT LINK BETWEEN DESIGN AND EXECUTION
At Projipharm Engineering, we do not consider quality to be a simple validation step at the end of a project. We integrate it as a common thread, ensuring that every technological choice, every technical specification and every phase of the study contributes directly to the operational and regulatory excellence of your future industrial tool.

📢 Need support to ensure your project’s compliance and performance? Contact our experts now!
Our Activities
Whether on a contract or fixed-price basis, Projipharm provides services to industrial companies. Our offering enables us to support our clients throughout the design and implementation phases.
Our expertise covers all areas of industrial production, processes, equipment and infrastructure.
Learn more
Looking for information?
We’re here to help !
Questions, answers, solutions: everything is here